

  |
|
郑永祥(1) 庞 飞(2) 摘 要 本研究之目的在於尝试自周边血液分离及纯化鸭淋巴细胞,探测其对各种淋巴细胞裂殖原刺激之反应,并藉以探讨黄麴毒素对鸭淋巴细胞转型作用之影响。 将EDTA抗凝之成熟产蛋菜鸭血,於4℃下,1300×g,离心30分钟,取Buffy coat,经等量PBS稀释後,置於Ficoll-Paque上层,於4℃下,200×g,离心25分钟後,取界面层可得鸭淋巴细胞再经PBS冲洗一次,RPMI清洗培养液冲洗两次,每次均於4℃,200g,离心10分钟。细胞经cytospin後制成抹片,以Quick stain染色计数,所得鸭淋巴细胞纯度可达95%以上,平均每10ml血液约可分离2×107细胞。取鸭淋巴细胞(8×105/ml)分别加入最终浓度为0、5、10、20、40或80μg/ml之裂殖原(mitogen),包括有PWM、PHA、PWA及BSS;经培养72小时後,以3H-thymidine (1μCi/well)并入法测定鸭淋巴细胞转型(lymphocyte transformation)反应。结果以PHA5及10μg/ml浓度下所测得CPM值高於其他裂殖原。 为探讨黄麴毒素对鸭淋巴细胞转型作用之影响,取鸭淋巴细胞(8×105 cells/ml),以PHA (100μg/ml)为阳性对照组,分别加入104、101或10-2ng/ml的黄麴毒素B1;为了解微粒体代谢作用对黄麴毒素毒性之影响,於各测试组中加入有或无鸭肝脏微粒体(1mg/ml)与NADPH (0.01mM)二组,经培养後,结果以黄麴毒素101及10-2ng/ml测得之CPM值显着高於其他各组(P<0.05),显示低浓度黄麴毒素对鸭淋巴细胞增殖具有刺激作用,轻微的抑制现象仅在高浓度104ng/ml下始出现。反之,当有微粒体存在时,即使在低浓度10-2ng/ml下对鸭淋巴细胞转型作用即有负面影响,而此抑制作用随黄麴毒素浓度升高而愈明显。因此,黄麴毒素在由肝脏微粒体的代谢後,可显着抑制PHA所引发的鸭淋巴细胞转型作用。 关键词:黄麴毒素,淋巴细胞转型作用,鸭。 ───────────────────────────────────── 1.国立宜兰农工专科学校畜产科讲师。 2.国立台湾大学兽医学系教授。 (1994年11月22日收件;1995年2月7日接受) 前 言 台湾的高温多湿,提供了饲料中霉菌生长极有利的条件,饲料中黄麴毒素污染问题一直是相当敏感的事情,虽然高剂量之黄麴毒素污染情形极少,但低量污染却仍然时有所闻(1)。 黄麴毒素乃黄麴菌中Aspergillus flavus及Aspergillus parasiticus之二次代谢产物。黄麴毒素题霉菌毒素中最重要之一种,为一群结构相似,自然存在之鶭喃氧杂鸻邻酮(furnanocoumarins)(7),曾造成1960年英国爆发10万只火鸡死亡,称之为火鸡不名疾病 (turkey 'X' disease)(3)。黄麴毒素除了一般人熟知之肝毒性及致癌性之外,尚具有免疫抑制性(20)。报告指出黄麴毒素具有抑制多种非特异性和特异性免疫反应及细胞免疫功能的作用(10),如抑制伴刀豆球蛋白所激发之淋巴细胞转型作用(2),降低鸡网状内皮系统清除碳粒能力(15),抑制鸡血液单核球之趋化性及扰乱固着性及游走性吞噬细胞的吞噬作用(6,19),甚至可进而造成疾病抵抗力的下降(21,24)。 综观上述黄麴毒素导致家畜禽免疫能力下降之研究大多集中於猪和鸡,但对鸭之研究则阙如。鸭虽具耐粗食和抗病力强等特性,但对黄麴毒素却甚为敏感(16),本实验之目的系尝试分离及纯化鸭淋巴细胞,并探讨黄麴毒素对鸭淋巴细胞经淋巴球裂殖原(mitogen)所激发的转型作用(lymphocyte transformation)之影响,以期对黄麴毒素对鸭免疫力之影响有初步的了解。 材料与方法 实验动物:以成熟的产蛋菜鸭做为血液供应鸭,采血期间鸭只采笼饲,饲料及饮水均自由采食。 鸭淋巴细胞之分离与纯化:采用之方法系依Higgins(9)所发表者予以修改。先自鸭翼下静脉采血,并以EDTA (0.2%)抗凝。该血液,经4℃,1300×g下离心30分钟,收集buffy coat,与含0.2% EDTA之等量1×PBS (pH7.2,41℃)混合。再将之置於等量Ficoll-Paque (density:1.077;Sigma, St Louis, Mo., USA)上於4℃,200×g下离心25分钟,将细胞层吸出,先以1×PBS清洗一次,於4℃,200×g下离心10分钟後再以含EDTA(0.2%)之RPMI-1640清洗两次,取得高纯度之淋巴细胞。除以trypan blue (0.2%)行vital stain测定细胞存活率外,并取适量细胞液以细胞抹片离心机(Shandon cytospin, Shandon Southern Instruments, Sewelicky, PA, USA)制成抹片,经Diff-Quick染色(International Reagents Corp, Japan)後於显微镜下计数200个细胞,测定细胞之分类。依上述结果,将细胞悬浮液以含20%胎牛血清、青霉素(100U/mL)+链霉素(100μg/mL)及1%L-glutamine之RPMI-1640调整最终细胞浓缩为1×107淋巴细胞/mL。 裂殖原之制备:本研究中选用PHA (phytohaemagglutinin)、PNA (peanut agglutinin)、PWM (pokeweed mitogen)及BSS (bandeiraea simplicifola seed)等四种裂殖原。先以含20%非动化胎牛血清之培养用RPMI-1640调整为5mg/mL之原备用。 肝脏微粒体之制备:取鸭肝脏40g置於200ml冰冷之1×PBS中,将肝脏剪碎。使用均质机(Ace homogenizer, Nissei AM-5)予以均质,再以组织研磨机(Wheaton overhead stirrer tissue)研磨来回三次。均质液以10000×g离心15分钟(HIMAC, CR21)使细胞核及粒线体沉淀,上清液以100000×g离心60分钟,倒去上清液後将沉淀之肝细胞微粒体,以1×PBS稀释为5mg/mL微粒体蛋白质之原液,微粒体蛋白质含量系依Lowry(13)所发表的方法修定测之,以胎牛血清做为蛋白质标准。 黄麴毒素B1制备:将纯品AFB1结晶50mg (Serva, Heidelberg, Germany),先以5mL二甲亚枫(dimethyl sulfoxide, DMSO)溶解,再以1×PBS调整为1mg/mL之原液,以铝箔纸密封遮光置於-20℃保存备用。 NADPH之制备:NADPH (Sigma, St. Louis, MO., USA),以1×PBS调整为0.25mM溶液,再以铝箔纸密封遮光置於-80℃保存备用。 试验一:裂殖原对鸭淋巴细胞的转型作用 实验中系使用U型之96孔微量平板(microplate),取80uL,1×107 cells/mL鸭淋巴细胞,加入含40%非动化胎牛血清之RPMI-1640 100μL和最终浓度10倍之裂殖原20μL,使各孔总体积为200μL中含淋巴细胞8×105 cells/mL,20%FBS和裂殖原。将微量平板置入41℃,含5%CO2之湿压恒温箱中培养72小时,络止培养前6小时加入含1μC i3Hthymidine (specific activity, 6.7mCi)之RPMI 20μL,细胞经细胞收集器(PHD Cell Harvester, Cambridge, TechnologyInc., Watertown, WA, USA)收集於玻璃纤维滤纸,滤纸置入含2ml闪烁液(0.5g bezene/toulene)之闪烁瓶中,以βcounter (LS 6000 IC, Beckman Inst. Inc., CA, USA)测定细胞所摄入的3H量,结果以CPM (count per minute)值表示。各处理组详如表1所示。 |
| Table l. The experimental design of the effect of various mitogens on duck lymphocyte transformation | ||||||
| Treatment | ||||||
| Lym1)+ mitogen 0μg/mL | Lym+ mitogen 5μg/mL | Lym+ mitogen 10μg/mL | Lym+ mitogen 20μg/mL | Lym+ mitogen 40μg/mL | Lym+ mitogen 80μg/mL | |
| RPMI
Lymphocytes (1×107 cells/mL) Mitogen* (200μg/ml) Total |
120μL
80μL
0μL
200μL |
115μL
80μL
5μL
200μL |
110μL
80μL
10μL
200μL |
100μL
80μL
20μL
200μL |
80μL
80μL
40μL
200μL |
40μL
80μL
80μL
200μL |
| *Mitogens included BSS、PHA、PNA、PWM.
1) Lym=Lymphocytes. |
||||||
|
试验二:黄麴毒素B1对鸭淋巴细胞转型作用的影响 取1×107 cells/mL之鸭淋巴细胞10μL置於微量平板中,除培养液、肝脏微粒体和对照组外其馀各组均加入25μL浓度为100μg/mL之PHA,并依所需於不同处理组中加入肝脏微粒体50μL及NADPH (0.25mM),和不同浓度之AFB1 (104、101、10-2 ng/mL) 45μL,并以RPMI调整使各孔之总体积为250μL、各处理组详如表2所示,其後之培养方法如同试验一。 |
| Table 2. The experimental design of the effects of AFB1 on PHA-induced duck lymphocytes transformation | |||||||||
| Treatment * | |||||||||
| CTRL- | CTRL+ | MIC | A4+MIC | A4 | A1+MIC | A1 | A-2+MIC | A-2 | |
| RPMI
Lymphocytes (1×107/mL) PHA (100μg/mL) AFB1 Microsome (5mg/mL) MADPH (0.25mM) Total |
150μL
100μL
-
- -
-
250μL |
125μL
100μL
25μL
- -
-
250μL |
90μL
100μL
-
- 50μL
10μL
250μL |
20μL
100μL
25μL
45μL 50μL
10μL
250μL |
80μL
100μL
25μL
45μL -
-
250μL |
40μL
100μL
25μL
45μL 50μL
10μL
250μL |
80μL
100μL
25μL
45μL -
-
250μL |
20μL
100μL
25μL
45μL 40μL
10μL
|
80μL
100μL
25μL
45μL -
-
|
| *CTRL-=medium control:CTRL+=PHA control:MIC=microsome alone; A4+MIC=AFB1 104 ng/mL+MICROSOME; A4=AFB1 104ng/mL; A1+MIC=AFB1 101ng/mL+microsome;A1=AFB1 101ng/mL;A-2+MIC=AFB1 10-2 ng/mL+MICROSOME;A-2=AFB1 10-2 ng/ml。 | |||||||||
|
统计分析: 实验所得之 资料先经一般线性式(general linear modle;GLM)进行变方分析,再以邓肯氏新多项变域法(Duncans new multiple range test)比较各组间差异显着性。 结果与讨论 鸭血液经Ficoll-Paque梯度处理,纯化後,细胞抹片计数可得到纯度为95±1.5%之淋巴球,单核球2.0±1.2%,颗粒球1.8±0.3%,红血球1.0±0.2%和血栓球0.5±0.1%。平均每10mL血液约可分离2×107细胞。红血球之污染在经低张溶液(0.5%,NH4C1)作用3分钟,均可完全分解,且对淋巴球之活力无影响。经由此法所分离之菜鸭淋巴细胞纯度与Higgins and Chung. (8)使用北京鸭分离之淋巴细胞纯度相似(83~93%)。但本试验中血液先经离心取得Buffy coat;故在Ficoll-Paque的用量较为节省。由於禽类血液的淋巴细胞在纯化上有些困难,包括高浓度颗粒球(4万个以上/μL);以及淋巴球大小与血栓球和红血球相似(14),以往的报告也因禽类淋巴细胞纯化效率差,且过程复杂,而大多仅用Buffy coat,进行禽类淋巴细胞生物学的研究,也因此间接阻碍了禽类免疫学的研究,较高纯度的鸭淋巴细胞取得,首先源自Boyum(5)之方法。将取得之全血与等量PBS混合,可降低血液粘度,改善分离效率。本试验以Buffy coat与等量PBS混合亦可达到降低粘度之效果并改善鸭周边血液淋巴细胞与Ficoll-Paque的相容性(compatability)。 有关不同裂殖原对鸭淋巴细胞转型作用之测试,其结果示於表3。表中显示BSS对鸭淋巴细胞转型反应最佳浓度为40μg/mL,PHAl为5μg/mL,PNA为40μg/mL,而PWM为5μg/mL。试验使用之裂殖原,除PHA及PHA之CPM超过40000以上,其馀BSS及PWM之CPM值均低於10000。此与Higgins.(9)之报告指出最佳反应浓度及CPM值分别为BSS 40μg/mL,23415;PHA 20μg/mL,4754; PNA 20μg/mL,6962;及PWM 40μg/mL,11297,有所差异。归纳其可能原因有:血清来源不同,本试验使用胎牛血清,而Higgins(9)使用成熟鸭血清;其次可能是血栓球的存在浓度,血栓球一般认为具有抑制淋巴细胞对裂殖原转型作用的反应(23)。此外,鸭种不同可能对裂殖原的反应亦不同,而此点可能系因特异受器的存在与否及存在的量有关。裂殖原可特异性的位於动物细胞表面糖蛋白及糖脂质内之单糖或寡糖,而淋巴细胞的转型作用不仅有赖适当的受器存在,另外该等受器分布的密度使其能与裂殖原形成交叉键结(cross-linking),亦是决定因子之一。故有些裂殖原虽与受器结合,但如受器密度低时,亦无转形反应产生(18)。由於菜鸭淋巴细胞胞对T细胞刺激原PHA及B细胞刺激原PNA有较佳的转型反应,但对於B及T细胞均有刺激作用的BSS及PWM的转型反应却不佳。由於PHA及PNA能与细胞糖蛋白及糖脂质之N-acetylgalactosamine结合,而此等受器在鸭淋巴球不仅存在且其分布的密度极适合PHA及PHA与之形成交叉链结,因而导致鸭淋巴细胞明显的有丝分裂的进行(8,9)。 |
| Table 3. Effects of various mitogens at different concentrations on duck blood lymphocyte transformation | ||||||
| 裂殖源 | Cpm values at various concentraction1) | |||||
| mitogen* | 0 | 5 | 10 | 20 | 40 | 80 |
| BSS
PHA PNA PWM |
828±230
853±218 736±244 763±210 |
3705±828
44565±2627 3916±1257 7260±1527 |
4603±787
39926±4579 6730±1662 6390±1630 |
3444±769
31020±4691 14213±2980 1586±344 |
5197±643
24748±5935 41068±6992 2269±1342 |
3585±1313
2845±972 2586±1011 765±322 |
| 1)
CPM:(count per minute).
*BBS:bandeiraea simplicifola; PHA:phytohaemagglutinin; PNA:peanut agglutinin; PWN:pokeweed mitogen. |
||||||
|
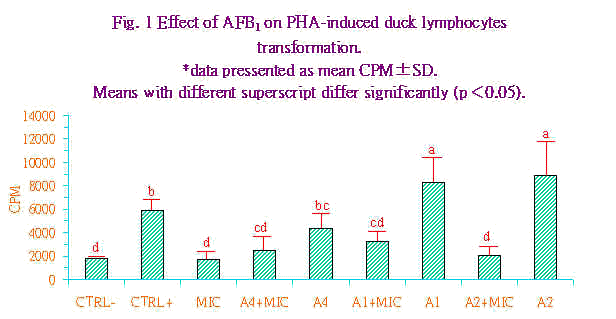
Higgins.(9)指出在低浓度之PHA有较佳之转型反应可能和受器的异质性(heterogenecity)有关。即当反应的裂殖原浓度改变时与糖脂质或糖蛋白质之亲和力或结合力(association)即改变。此外,在反应的细胞群中分化细胞携带有不同的接受器,故对裂殖原有不同的反应。 有关AFB1对鸭淋巴细胞转型作用之影响,其结果示於图1。图中显示AFB1在低浓度时(101及10-2ng/mL)对由PHA所引发的鸭淋巴细胞转型作用具有刺激的效果,且显着较PHA阳性处理组为高(P<0.05),反观当AFB1的浓度提升至104ng/mL时,即开始对PHA所引发的鸭淋巴球转型作用又有抑制的趋势,虽然此抑制效果在统计学上并不显着。然而当各AFB1组中加入肝脏微粒体後,无论是101或是10-2均对PHA所引发的鸭淋巴球转型作用产生极显着的抑制作用。由以上的结果显示肝脏微粒体在AFB1对鸭淋巴球转型作用抑制性上扮演重要的加乘效应,此点和AFB1对火鸡腹腔巨噬细胞的毒性相类似(17)。AFB1如未经肝脏微粒体的活化,即使在高浓度下40μg,对火鸡腹腔巨噬细胞的附着力、形态学,及吞噬能力均无显着的影响;反之,经肝脏微粒体代谢後,AFB1在极低的浓度下(1~5μg)即可造成火鸡腹腔巨噬细胞附着力和吞噬能力的明显下降,且细胞表面呈球状突起而核亦呈分解状(17)。AFB1在氧和NADPH的存在下,可被仰赖cytochrome P450的肝脏微粒体mixed function oxidase (MFO)系统所活化(12),其中一个代谢产物,2,3-oxide可和DNA上N7-guanine结合而产生细胞毒性、致癌性及畸胚性等影响(11)。Bodine et al. (4)亦曾指出受裂殖原PHA致活之牛淋巴球能将AFB1进一步代谢为可和DNA结合的细胞突变物质。且抑制活体外淋巴细胞对各种抗原或细胞裂殖原的刺激反应(21,24)。 |
|
综合本试验之结果显示PHA对菜鸭淋巴球有较佳的转型反应,在无肝脏微粒体存在下低浓度AFB1对该转型反应具有刺激作用,但AFB1经肝脏微粒体代谢後对转型反应具有抑制作用。由於鸭较其他动物对AFB1具敏感性;利用鸭只此一特性探讨AFB1对其生理与免疫反应的影响,应是颇佳之动物模式。 志 谢 本试验承蒙国立台湾大学畜产学系陈保基教授提供试验鸭只,特此致射。 参考文献 1.吴继芳、郑清森、游义德、严家清、郭忠政。1988。饲料中含低浓度黄麴素对生长猪生长性能与组织残留量之影响。中华农学会报. 144:85-93 2.庞飞。1994。黄麴毒素对猪只免疫力影响之研究。生命科学简讯。8(3):1-9. 3.Blount, W. P. 1961. Turkey X disease. J. Brit. Turkey Federation. 9:52. 4.Bodine, A. B., S. F. Fisher and S. Gangjee. 1984. Effect of aflatoxin B1 and major metabolites on phytohemagglutinin stimulated lymphoblastogenesis of bovine lymphocytes. J. Dairy Sci. 67:110-114. 5.Boyum, A. 1968. Isolation of leucocytes from human blood. A two phase system for removal of red cells with methyl cellulose as erythrocyte-aggregatting agent. Scand. J. Clin. Lab. Invest. 21 (suppl. 9),4. 6.Chang, C. F. and P. B. Hamilton. 1979. Impaired phagocytosis by heterophils from chickens during aflatoxicosis. Toxicol. Appl. Pharmacol. 48:459-466. 7.Ciegler, A. and E. B. Lillehoj. 1968. Mycotoxins. Advan. Appl. Microbiol. 10:155-219. 8.Higgins, D. A. and S. H. Chung. 1986. Duck Lymphocytes: I. Purification and preliminary observations on surface markers. J. Immunol. Methods. 86:231-238. 9.Higgins, D. A. 1990. Duck Lymphocytes: III. Transformation responses to some common mitogens. Comp. Immunol. Microbiol. Infect. Dis. 13:12-23. 10.Kadian, S. K., D. P. Monga and M. C. Goel. 1988. Effect of aflatoxin B1 on the delayed type hypersensitivity and phagocytic activity of reticuloendothlial system in chickens. Mycopathologia 104:33-36. 11.Karenlampi, S. O. 1987. Mechanism of cytotoxicity of aflatoxin B1: Role of cytochrome P1-450. Biochem. Biophys. Res. Commun. 145:854-860. 12.Koser, P. L., M. B. Faletto, A. E. Maccubbin, and H. L. Gurtto. 1988. The genetics of aflatoxin B1 metabolism, Association of the induction of aflatoxin B1-4-hydroxylase with the transcriptional activation of cytochrome P3-450 gene. J. Biol. Chem. 263:12584-12595. 13.Lowry, O. H., N. J. Rosebrough., A. L. Far and R. J. Randall. 1951. Protein measurement with folin phenol reagent. J. Biol. Chem. 193:265-275. 14.Lucas, A. M. and C. Jamroz, 1961. Atlas of Avian Hematology, Agriculture Monograph 25 (U.S. Department of Agriculture, Washington, DC). 15.Michael, G. Y., P. Thaxton and P. B. Hamilton. 1973. Impairment of the reticuloendothelial system of chickens during aflatoxicosis. Poult. Sci. 52:1206-1207. 16.Muller, R. D., C. W. Carlson, G. Semeniuk and G. S. Harshfiled. 1970. The response of chicks, ducklings, goslings, pheasants and poults to graded levels of aflatoxins. Poult. Sci. 49:1346-1350. 17.Neldon-Ortiz, D. L. and M. A. Qureshi. 1991. Direct and microsomal activated aflatoxin B1 exposure and its effects on turkey peritoneal macorphage functions in vitro. Toxicol. Appl. Pharmacol. 109:432-442. 18.Pearson, T. W., G. E. Roelants, L. B. Lundin and K. S. Mayor-Withey. 1979. The bovine lymphoid system:binding and stimulation of peripheral blood lymphocytes by lectins. J. Immunol. Methods 26:271-275. 19.Richard, J. L. and J. R. Thurston. 1975. Effect of aflatoxin on phagocytosis of Aspergillus fumigatus spores by rabbit alveolar macrophages. Appl. Microbiol 30:40-47. 20.Pier, A. C., S. J. Cysewski. J. L. Richard, and J. R. Thurston. 1977a. Mycotoxins in human and animal health. Edited by J. V. Rodricks, C. W. Hesseltine, and M. A. Mehlman. Park Forest South, Illinois, Pathotox Publ. pp. 745-750. 21.Singh, J., R. P. Tiwari, G. Singh, S. Singh and D. V. Vadehra. 1987. Biochemical and immunological effects of aflatoxins in rabbits. Toxicol. Letters 35:225-230. 22.Thurston, J. R. M., B. L. Royoe, A. L. Baetz, J. L. Rchard and G. D. Booth. 1974. Effect of aflatoxin of serum protein, complement activity, and the antiboby response to Brucellla abortus in guinea pigs. Am. J. Vet. Res. 35: 1097-1100. 23.Traill, K. N. G. Bock, R. Boyd, G. Wick. 1983. Chicken thrombocytes. Isolation, serological and functional characterisation using the fluorescense activated cell sorter. Dev. Comp. Immunol. 7:112-115. 24.Yang, W. C., 1983. Effects of aflatoxin B1 on the development of procine cellar and humoral immune responses. Ph. D. dissertation, Auburn University, Alabama, USA. |
|
Effects of Aflatoxin B1 (AFB1) on Duck Lymphocyte Transformation Yeong-Hsiang Cheng(1) and Victor-Fei Pang(2) Summary The purpose of this experiment was to isolate and purify duck lymphocytes from peripherial blood. Lymphocytes were then used to study transformation response to various mitogens. These results were used to evaluate the effects of aflatoxin on duck lymphocyte transformation. The buffy coat collected from the whole blood was anticoagulated with EDTA andcentrifuged at 1300×g for 30min at 4℃, then resuspended with an equal wolume of PBS. The cell suspension was layered over a Ficoll-Paque gradient and centrifuged at 200×g for 25min at 4℃. The band formed at the interface of the RBC layer contained cells of which greater than 95% were lymphocytes. The averaged numbers of lymphocytes obtained from 10 mL of whole blood wasapproximately 2 ×107. Various mitogens (PWM, PHA, PNA, and BSS) at a final concentration of 0,5,10,20,40and 80μg/mL were added to the lymphocyte suspension (8×105 cells/mL). The Cpm value of the lymphocyte transformation response by 3H-thymidine integration after 72hrs incubation was measured. The results showed that PHA at 5 and 10μg /mL gave higher values than the others. The effects of aflatoxin on duck lymphocyte transformation response were evaluated, by addition of final concentration of 104,101, or 10-2 ng/mL aflatoxin B1 (AFB1) to lymphocyte suspension. Meanwhile, lymphocyte suspension with or without duck liver micorosome plus NADPH was also evaluated. The results showed AFB1 101 and 10-2ng/mL had significantly higher CPM values than the others (P<0.05). This indicated that AFB1 had a stimulative effect on duck lymphocyte transformation. AFB1 only at 104ng/mL had a slight inhibition. When liver microsome was present, AFB1 had an adverse effect on duck lymphocyte transformation even though at a low concentraction of 10-2ng/mL. This detrimental effect became more obvious as AFB1 concentration increased. It could thus be concluded that PHA-induced duck lymphocyte transformation was inhibited significantly when AFB1 was metabolized by duck liver micorsome, Key words:Aflatoxins, Lymphocyte Transformation, Duck ──────────────────────────────────── 1.Department of Animal Science, National I-Lan Institute of Agriculture and Technology, I-Lan Taiwan, R.O.C. 2.Department of Veterinary Medicine, National Taiwan University, Taipei, Taiwan, R.O.C. (Received November 22, 1994; Accepted February 7,1995)
|