

  |
|
表一 以MTT法测定黄麴毒素B1对鸭淋巴细胞毒害作用之试验处理 Table 1. The experimental design of cytotoxic effect of aflatoxin B1 on duck lymphocytes by MTT assays |
|||||||||
| CTRL *- | CTRL+ | MIC | AFB1 104 ng/ml MIC | AFB1 104 ng/ml | AFB1 101 ng/ml MIC | AFB1 101 ng/ml | AFB1 10-2 ng/ml MIC | AFB1 10-2 ng/ml | |
| RPMI-1640
Lymphocytes (1×107/ml) AFB1 PHA (200μg/ml) MIC (12.5 mg/ml) NADPH (0.25 mM) Total |
200
50
- -
-
-
250 |
175
50
- 25
-
-
250 |
170
50
- -
20
10
250 |
100
50
45 25
20
10
250 |
130
50
45 25
-
-
250 |
100
50
45 25
20
10
250 |
130
50
45 25
-
-
20 |
100
50
45 25
20
10
250 |
130
50
45 25
-
-
250 |
| *CTRL-=medium control;CTRL+=PHA alone;MIC=microsome alone;Volume unit=μl. | |||||||||
| 试验四:培养系统加入不同最终浓度之β-胡萝卜素(0.025、0.05、0.1μM)和添加肝脏微粒体与黄麴毒素B1 (1×104 ng/ml),最终细胞浓度为1×107淋巴细胞/ml,以探讨黄麴毒素B1对鸭周边血液淋巴细胞之致害作用及β-胡萝卜素对其是否具保护作用。试验处理详如表2。 |
|
表二 以 MTT 法 测 定 黄 麴 毒 素 B1 对 鸭 周 边 血 液 淋 巴 细 胞 之 致 害 作 用 及 β- 胡 萝 卜 素 对 其 保 护 效 果 之 验 处 理 Table 2. The experimental design of cytotoxic effect of aflatoxin B1 on duck lymphocytes and protective activity of β-carotene by MTT assays |
|||||||||
| CTRL- | CTRL+ | MIC | BCH | AFB1 104 ng/ml | AFB1 104 ng/ml MIC | AFB1 104 ng/ml MIC+ BCL | AFB1 104 ng/ml MIC+ BCM | AFB1 104 ng/ml MIC+ BCH | |
| RPMI-1640
Lymphocytes (1×107/ml) AFB1 PHA (200μg/ml) betacarotene MIC (12.5 mg/ml) NADPH (0.25 mM) Total |
200
50
- -
- -
-
250 |
175
50
- 25
- -
-
250 |
170
50
- -
- 20
10
250 |
170
50
- -
30 -
-
250 |
130
50
45 25
- -
-
250 |
100
50
45 25
- 20
10
250 |
70
50
45 25
30 20
10
250 |
70
50
45 25
30 20
10
250 |
70
50
45 25
30 20
10
250 |
| *CTRL-=medium control;CTRL+=PHA alone;MIC=microsome alone;BCH=betacarotene alone (0.1μM);Volume unit=μl. | |||||||||
|
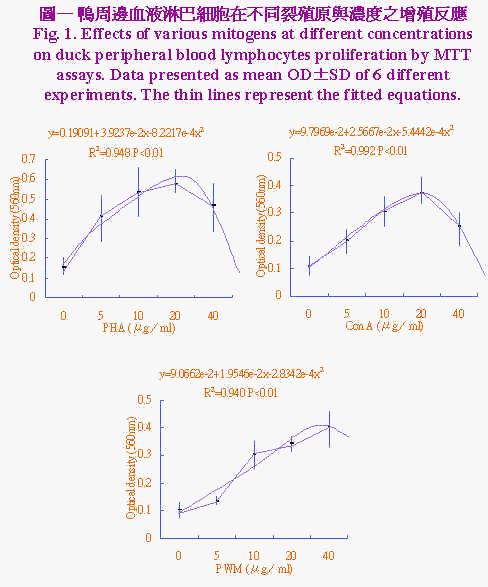
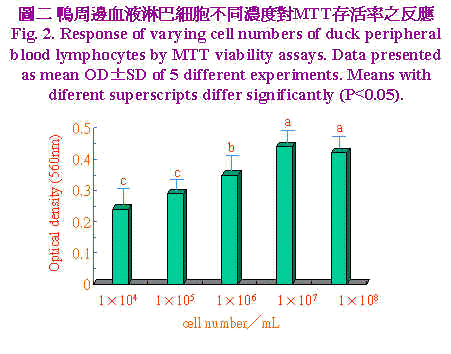
三、统计分析 实验所得之资料先经一般线性模式(general linear model;GLM)进行变方分析,再以邓肯氏新多项变域法(Duncan's new multiple range test)比较各组间差异显着性。 结果与讨论 不同裂殖原PHA、Con A及PWM於不同浓度(0、5、10、20和40μg/ml)加入培养系统中,以探讨最佳MTT反应之裂殖原与浓度,结果示於图1。资料经多项式(polynominal)回归所绘之回归曲线图,由曲线图可知裂殖原以PHA刺激增殖反应最佳,PWM次之,而ConA最差。回归方程式经一次微分求得增殖反应所须最大裂殖原浓度分别为PHA 23.86μg/ml、Con A 23.57 μg/ml和PWM 34.48 μg/ml。鸭周边血液淋巴细胞不同浓度对MTT存活率之反应,示於图2淋巴细胞数对MTT之反应呈二次曲线,当细胞浓度自1×104提高至1×107 cell/ml时,淋巴细胞数目持续增加其OD值亦升高,当细胞浓度再增加到1×108 cell/ml时OD值呈降低的趋势,推测此一细胞浓度在经72小时培养後,营养分可能为其增殖之限制因子,亦即胎牛血清已用尽,由此一结果显示以MTT方法测定鸭淋巴细胞之存活率以1×107 cell/ml最为适当,此一结果与Lessard and Dupuis (1994)试验使用之细胞数一致。不同家畜禽之淋巴细胞表面之裂殖原受体数目与特性不一,对同一裂殖原反应时之最佳细胞数互异(Talebi et al., 1995)。 |
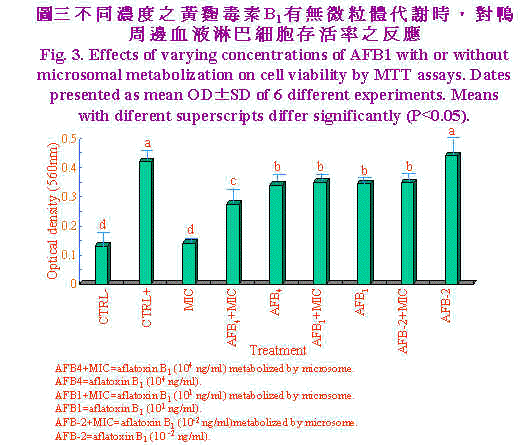
| 以不同浓度之黄麴毒素B1经有无微粒体代谢时,对鸭周边血液淋巴细胞存活率之影响,结果示於图3。单独淋巴细胞或微粒体处理淋巴细胞增生数目相似,显示微粒体对其并无刺激效果,但当PHA (20μg/ml)加入时,淋巴细胞受其刺激而大量增殖。黄麴毒素B1自高量(1×104 ng/ml)至低量时(1×101 ng/ml)OD值显着(P<0.05)较单独PHA处理者为低,此两浓度显然已造成淋巴细胞存活率之降低,但黄麴毒素B1 (1×10-2 ng/ml)时对淋巴细胞增殖无抑制作用,可能为低量黄麴毒素且经淋巴细胞代谢後产生之毒性代谢物低至不影响其增殖作用。当高浓度黄麴毒素B1 (1×104 ng/ml)经微粒体代谢後,显着(P<0.05)抑制淋巴细胞增殖作用,由本试验结果显示黄麴毒素B1 (104 ng/ml)经代谢後可产生淋巴细胞致害作用,但浓度降低(1×101、1×10-2 ng/ml)时致害作用不显着。 |
|
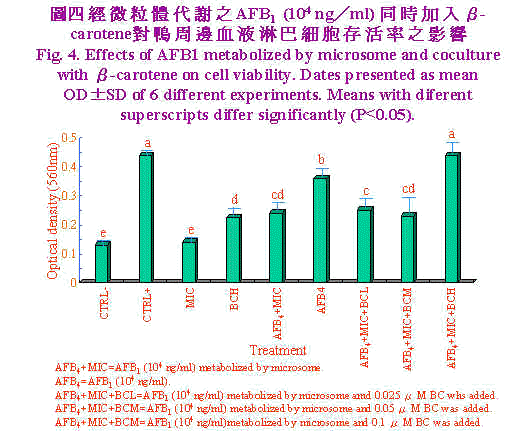
肝脏微粒体为细胞经均质化裂解而来,内质网断裂後,再组合而成大小约100nm之小泡(Forrester et al., 1990),其富含多种酵素,其中以cytochrome p450在黄麴毒素B1代谢转化为8,9-epoxide毒性增强(Ramsdell and Eation, 1990)。Neldon-Ortiz and Qureshi (1991)以经微粒体代谢之黄麴毒素B1作用鸡腹腔巨噬细胞1小时,则发现微粒体使黄麴毒素B1毒性增强,甚至0.5μg/ml之浓度亦造成细胞形态改变,黏附力及吞噬能力亦明显降低。相较本试验使用之浓度104 ng/ml等於10 μg/ml,意即以淋巴细胞存活率来评估黄麴毒素B1经代谢之毒性上比以巨噬细胞之形态学、黏附力及吞噬力为评估指标较不敏感。Pang and Pan (1994)以猪淋巴细胞为对象,结果显示黄麴毒素B1浓度於5×104 ng/ml处理12小时,猪淋巴细胞死亡率即达50%。 经微粒体代谢之AFB1 (104 ng/ml)同时加入β-胡萝卜素(BC)对鸭周边血液淋巴细胞存活率之影响,结果示於图4。单独微粒体处理与培养基对照组间无显着差异,而当单独高浓度BC (0.1 μM)添加时,OD值显着(P<0.05)较培养基对照组为高,显示BC为一潜在性裂殖原。单独AFB1 (104 ng/ml)显着(P<0.05)较AFB1经微粒体代谢者之细胞存活率为高,且中低浓度BC (0.05、0.025 μM)添加於AFB1经微粒体代谢之培养系统中时对细胞存活率并无改善之效果。然而高浓度BC (0.1 μM)添加於AFB1经微粒体代谢之培养系统中时极显着的(P<0.05)较中低浓度BC者之细胞存活率为高,且改善至单独PHA对照组相同之细胞存活率。 Chew et al., (1993b)实验指出仔牛一次口服胶囊化BC 200 mg时,可提高淋巴细胞对BC之摄取量,但白血球及红血球对BC摄取量无影响。进一步分析淋巴细胞之胞器。发现粒线体、胞核及微粒体中BC含量着增加,但仔牛血浆中BC及视觉醇含量无影响。本试验之BC (0.1 μM)添加可有效的提高淋巴细胞存活率,可能与提高淋巴细胞对BC之摄取有关;虽然对於BC在调节免疫力上所扮演的角色尚不了解,但BC可能以自由基清除者抵抗自由基导致之细胞受损。事实上,生体内和生体外研究指出,BC可促进淋巴细胞增殖作用(Hoskinson et al., 1989)及增加助手T细胞数目(Prabhala et al., 1989)。而本试验之淋巴细胞含高浓度BC可能保护或对抗AFB1经代谢後产生之自由基,此外BC也可能直接调节淋巴细胞之增殖。Chew et al., (1993b)实验发现视觉醇并未随之升高,显示BC有别於一般人认为仅是维生素A原之刻板印象,而是有其独特的作用。 虽然AFB1造成淋巴细胞死亡的确实机制未知,但AFB1经微粒体酵素代谢之二次产物造成细胞膜不稳定可能扮演一重要地位(Pokrovsky et al., 1972)。曾有报告指出,氧自由基造成细胞膜受损,而致脂质过氧化、蛋白质及DNA之破坏(Perera et al., 1987)。近来研究显示AFB1及其代谢物可经酵素非酵素作用形成自由基(Kodama et al., 1990),是否自由基为造成淋巴细胞存活率降低之主因,须加以进一步证实。Lessard and Dupuis (1994)生体试验也陈述日粮中BC含量增加,鸡淋巴细胞增殖作用及自然杀手细胞(natural killer)活性亦提高。但亦有试验指出BC或BC与维生素E协同时无法提高以E. coli免疫鸡只的凝集抗体力价,维生素E或A却可以提高,此可能与BC对疾病保护机制异於维生素E或A有关。 生体外试验,当添加BC浓度为10-8M对鼠脾脏淋巴细胞增殖无影响,添加BC10-5M抑制牛周边血液淋巴细胞之增殖作用(Tjoelker et al., 1988),此一结果与本试验结果相去甚远,其原因可能为不宣品种动物对BC摄取程度差异及free radical存在与否时造成之影响。此一推论基础可由Lawlor and O'brien (1995)应用paraquat 0.25 mM处理鸡胚纤维母细胞(chicken embryo fibroblast, CEF)18小时,造成其氧化性紧迫,当0.1μM BC添加时,superoxide dimutase及glutathione peroxidase (GSH-px)活性回复至与对照组相同水准,且降低catalase活性。当10μM BC添加时superoxide dimutase及catalase显着升高且GSH-px活性降低至与对照组相当。显示BC有效保护CEF对抗paraquat诱发之氧化性紧迫。 综观本试验结果显示,高浓度AFB1 (104 ng/ml)经代谢後对鸭淋巴细胞毒性增强,高浓度胡萝卜素(0.1 μM)可保护鸭淋巴细胞免除此一毒害作用,是否为抑制氧自由基之产生,须进一步探讨。 |
|
参考文献 郑永祥、庞飞。1995。黄麴毒素B1对鸭淋巴细胞转型作用之影响。中华农学会报170:147~155。 Bendich, A., and S. S. Shapiro. 1986. Effect of β-carotene and canthaxanthin on the immune responses of the rat. J. Nutr. 116: 2254~2261. Bendich, A. 1991. Carotenoids and immunity Clin. App. Nutr. 1: 45~53. Bounous, D. I., R. P., Campagnoli and J. Brown. 1992. Comparoson of MTT colorimetric assay and titrated thymidine uptake for lymphocyte proliferation assay using chicken splenocytes. Avian Disease 36: 1022~1027. Chew, B. P., 1993a. Role of carotenoids in the immune response. J. Dairy Sci. 76: 2804~2811. Chew, B. P., T. S. Wong and J. J. Michal. 1993b. Uptake of orally administered β-carotene by blood plasma, leukocytes, and lipoproteins in calves. J. Anim. Sci. 71: 730~739. Denizot, F. and R. Lang. 1986. Rapid colorimetric assay for cell growth and survival modification to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Method 89: 271~277. Forrester, L. M., G. E. Neal, D. J. Judah, M. J. Galancey and C. R., Wolf. 1990. Evidence of involvement of multiple forms of cytochrome P-450 in aflatoxin B1 metabolism in human liver. Proc. Natl. Acad. Sci. 87:8306~8310. Hoskinson, C. D., B. P. Chew and T. S. Wong. 1989. Effects of β-carotene and vitamin A on mitogen-induced lymphocyte proliferation in the pig in vivo. FASEB J3:A663. Kadian, S. K., D. P. Monga and M. C. Goel. 1988. Effect of aflatoxin B1 on the delayed type hypersensitivity and phagocytic activity of reticuloendothlial system in chickens . Mycopathologic 104 :33~36. Kodama, M., F. Inoue and M. Akao. 1990. Enzymatic and non-enzymatic formation of free radicals from aflatoxins B1. Free Rad. Res. Comms. 10: 137~142. Lawlor, S. M. and N. M. O'brien. 1995. Modulation of oxidative stress by β-carotene in chicken embryo fibroblasts. Brit. J. Nutr. 73: 841~850. Lessard, M. and M. Dupuis. 1994. Differential modulation of chicken lymphocyte blastogenesis and cytotoxic activity of natural killer cells in vitro by retinol, retionic acid and beta-carotene. Nutr. Res. 14:1201~1217. Lowry, O. H., N. J. Rosebrough, A. L. Far and R. J. Randall. 1951. Protein measurement with folin phenol reagent J. Biol. Chem. 193: 265~275. Mosmann, T. 1983. Rapid colorimetric assay for cell growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Method 65:55~63. Neldon-Ortiz, D. L. and M. A. Qureshi. 1991. Direct and microsomeal activated aflatoxin B1 exposure and its effects on turkey peritoneal macrophage functions in vitro. Toxicol. Appl. Pharmacol. 109: 432~442. Pang, V. F. and C. Y. Pan. 1994. The cytotoxic effects of aflatoxin B1 on swine lymphocytes in vitro. J. Chin. Soc. Vet. Sci. 20(4):289~301. Perera, M. I. R., J. M., Bestschart, M. A. Virji, S. L. Katyal, H. Shinozuka. 1987. Free radical injury and liver tumor promotion. Toxicol. Pathol. 15:51~59. Peterson, G. L. 1977. A simplification of the protein assay method of Lowery et al. which is more generally applicable. Anal. Biochem. 83: 346~356. Pier, A. C., R. E. Fichtner and S. J. Cysewski. 1977. Effect of aflatoxin on the cellular immune system. Ann. Naer. Alim. 31:781~788. Pokrovsky, A. A., L. V. Kravchenko and V. A. Tutelyan. 1972. Effect of aflatoxin on rat liver lysosomes. Toxicon. 10:25~30. Prabhala, R. H., V. Maxey, M. J. Hicks and R. R. Watson. 1989. Enhancement of the expression of activation markers of human peripheral blood mononuclear cells by in vitro culture with retinoids and carotenoids. J. Leukocyte Biol. 45: 249~256. Pamsdell, H. S. and D. L. Eaton. 1990. Species susceptibility to aflatoxin B1 carcinogenesis: Comparative kinetics of microsomal biotrans formation. Cancer Res. 50: 615~620. Talebi, A., P. R. Torgerson and G. Mulcahy. 1995. Optimal conditions for measurement of blastogenic reponses of chickens to concanavalin A in whole blood assays. Vet. Immunol. and Immunopathol. 46: 293~301. Tengerdy, R. P., N. G. Lacetera and C. F. Nockels. 1990. Effect of beta carotene on disease protection and humoral immunity in chickens. Avian disease 34: 848~854. Tjoelker, L. W., B. P. Chew, T. S. Tanaka and L. R. Daniel. 1988. Bovine vitamin A and β-carotene in take and lactational status. 2. Responsiveness of mitogenstimulated peripheral blood lymphocytes to vitamin A and β-carotene challenge in vitro. J. Dairy Sci. 71: 3120~3127. |
|
The Cytotoxic Effect of Aflatoxin B1 on Duck Lymphocytes and the Protective Effect of β-Carotene Yeong-Hsiang Cheng(1) and Victor-Fei Pang(2) Received Oct. 26, 1995; Accepted Jan. 29, 1996 ABSTRACT The purpose of this experiment was to determine duck peripheral blood lymphocyte proliferation by a MTT (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. This assay was also used to evaluate the cytotoxic effect of aflatoxin B1 (AFB1) on duck lymphocyte and the protective effect of β-carotene (BC). Trial 1: Three mitogens were examined and the results showed that phytohaemagglutinin (PHA) had the best proliferation response, pokeweed mitogen (PWM) intermediate, and Concanavalin A (Con A) the least. Using best-fit regression, the best mitogen concentrations for proliferation response were PHA, 23.68 μg/ml;Con A, 34.48μg/ml and PWM, 23.57 μg/ml, respectively. Trial 2: The proliferation response of lymphocyte number by a MTT assay showed a quadratic fashion. When cell numbers were raised from 1×104 to 1×107 cell/ml, the OD values were also increased. However, the OD values tended to decrease when cell numbers were increased to 1×108 cell/ml. Trial 3:The results showed that AFB1 treatment from high dose (1×104 ng/ml) to low dose (1×101 ng/ml) had OD values significantly lower (P<0.05) than PHA treatment alone. But, there was no inhibitory effect when AFB1 Aat an extremly low dose (1×10-2 ng/ml) was introduced. High dose of AFB1 which was metabolized by microsome inhibited lymphocyte proliferation to a greater extent than AFB1 which was without microsome metabolization. There was no significant inhibition at low dose (1×101,1×10-2 ng/ml) treatments. Trial 4: The results showed that when high BC level (0.1μM) was added, OD values of lymphocyte proliferation were higher (P<0.05) than medium control. Treatment of AFB1 alone had higher (P<0.05) proliferation than AFB1 metabolized by microsome. There was no improvement on lymphocyte proliferation when medium and low levels of BC (0.05, 0.025 μM) were added to culture system of AFB1 metabolized by microsome. However, when high level of BC was added to the same culture system, the proliferation was higher (P<0.05) than medium and low levels of BC and the improvement was the same as PHA control group. This experiment revealed that high dose of AFB1 metabolized by microsome had an intensified effect on inhibition of duck lymphocyte proliferation. However, high levels of BC could alleviate this detrimental effect on duck lymphocyte. (Key Words: Duck, Aflatoxin B1, Lymphocyte, β-Carotene) ─────────────────────────────────── (1)Department of Animal Science, National 1-Lan Institute of Agriculture and Technology, I-Lan, Taiwan, R.O.C. (2)Department of Veterinary Medicine, National Taiwan University, Taipei, Taiwan, R.O.C. |